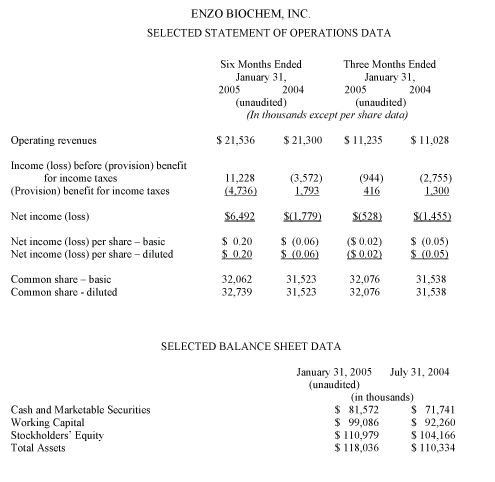
Fiscal first half revenues totaled $21.5 million, compared with $21.3 million in the corresponding period a year ago. Income before taxes, including the initial $14.0 million settlement gain recorded upon signing of the agreement with Digene in October 2004, amounted to $11.2 million, compared with a loss of $3.6 million in the corresponding year-ago period. Net income for the first six months of fiscal 2005 amounted to $6.5 million, or $0.20 per diluted earnings per share, compared with a net loss of $1.8 million, or ($0.06) per diluted share, a year earlier.
“The second quarter of fiscal 2005 showed solid progress,” said Barry Weiner, President of Enzo. “Enzo Clinical Labs had an excellent performance, with strong revenue growth. Enzo Life Sciences recorded its first royalty income under the Digene Corporation settlement. Overall revenues, however, were adversely affected by a determination not to recognize revenues from Roche Diagnostic Systems because of ongoing litigation, as compared to a year ago when full revenues were recognized. In addition, Enzo Therapeutics continued to move ahead with several clinical trials, including a newly initiated Phase II investigation of treatment for non-alcoholic steatohepatitis. The second fiscal quarter, overall, was a productive one, with favorable momentum.”
For the three months ended January 31, 2005, total revenues amounted to $11.2 million, compared with $11.0 million in the corresponding year-ago period and $10.3 million in the preceding first quarter of the year. The loss before taxes declined to $0.9 million, from $2.8 million a year ago. The second fiscal quarter net loss declined to $0.5 million, compared with a year-ago loss of $1.5 million, an improvement of $1.0 million. Diluted loss per share equaled ($0.02), compared to ($0.05) a year earlier.
Results for the second fiscal quarter of 2005 reflected a reduction in the provision for uncollectible receivables to $1.1 million, from $3.1 million a year earlier, and reduced legal expenses of $1.2 million, compared to $1.8 million a year ago. Research and development expenses for the quarter were also lower, totaling $2.0 million, compared with $2.3 million, due to the timing of certain clinical-related activities. The increase in selling, general and administrative expenses of $1.0 million principally reflected increased expenses in connection with the Company’s continued expansion of the marketing and information technology programs for both Enzo Labs and Enzo Life Sciences.
Second fiscal quarter revenues at Enzo Clinical Labs increased to $8.0 million, from $7.1 million a year ago, an increase of 13%. Gross profit increased to $5.1 million, compared with $4.5 million a year ago. Other costs and expenses amounted to $4.2 million, compared with $5.6 million a year ago. Operating income amounted to $0.9 million, compared to a year-ago operating loss of $1.1 million, a $2.0 million improvement, and was up from the fiscal 2005 first quarter of $0.6 million.
At Enzo Life Sciences, total revenues, including Digene royalties for the quarter, totaled $3.3 million, compared with $4.0 million in the corresponding year-ago quarter. The year-ago quarter included revenue from Roche, but as noted in light of recent litigation, current Roche revenues are not being recorded. Sequentially, Life Sciences revenues were up from $2.5 million in the first fiscal quarter of 2005, reflecting both the Digene royalties and direct sales. Direct sales of Life Sciences products, including those for amplification and labeling for both low and high density arrays, particularly the GeneBeam™ line, increased year-over-year.
Enzo Biochem’s balance sheet remains strong. As of January 31, 2005, cash, cash equivalents and investments totaled $81.5 million. Working capital exceeded $99 million, and shareholder’s equity was over $110 million. The Company remains debt-free.
Enzo Therapeutics Activity
Enzo Therapeutics is aggressively pursuing its drug development programs. The expanded study of Alequel™, the Company’s therapeutic modality for management of Crohn’s disease is underway and subjects are continuing to be enrolled. A clinical trial of EGS21, one of Enzo’s proprietary drug compounds, was initiated last week for the treatment of non-alcoholic steatohepatitis (NASH or fatty liver) and subjects are currently being enrolled. A Phase I safety trial of EGS21 was successfully concluded last year. This study is being partially funded by a $1.0 million grant from the Israel-U.S. Binational Industrial Research and Development Foundation (BIRD). The Company has received approval to move forward with clinical trials to evaluate the effect of EGS21 in the treatment of other conditions. A Phase II study will compare EGS21 and Alequel™ in the treatment of Crohn’s disease and will begin shortly. A Phase II trial to study the effect of EGS21 in the treatment of HCV-associated chronic active hepatitis has also been approved. In addition, Enzo has received approval to begin a Phase I trial to evaluate the use of oral immune regulation for the management of individuals suffering from rheumatoid arthritis who have been treated with Remicade® and have developed antibodies against Remicade®. The Phase I/II clinical study of HGTV-43™, Enzo’s gene medicine for HIV-1 infection is proceeding with the opening of another testing site. In addition, the Company has a number of new compounds in preclinical development that could provide therapy for treating bone disorders including osteoporosis, bone loss, fractures, abnormalities, diseases, and other applications.
About Enzo
Enzo Biochem is engaged in the research, development and manufacture of innovative health care products based on molecular biology and genetic engineering techniques, and in providing diagnostic services to the medical community. The Company’s proprietary labeling and detection products for gene sequencing and genetic analysis are sold to the life sciences market throughout the world. The Company’s therapeutic division is in various stages of clinical evaluation of its proprietary gene medicine for HIV-1 infection and its proprietary immune regulation medicines for hepatitis B and hepatitis C infection and for Crohn’s disease. The Company also holds a patent covering a method and materials for correcting point mutations or small insertions or deletions of genetic material that would allow for editing and correcting certain abnormalities in genes. The Company owns or licenses over 230 patents worldwide. For more information visit our website www.enzo.com.
Except for historical information, the matters discussed in this news release may be considered ”forward‑looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements include declarations regarding the intent, belief or current expectations of the Company and its management. Investors are cautioned that any such forward‑looking statements are not guarantees of future performance and involve a number of risks and uncertainties that could materially affect actual results. The Company disclaims any obligations to update any forward-looking statement as a result of developments occurring after the date of this press release.
An informational call conducted by Enzo Biochem, Inc. management will take place on Tuesday, March 15, 2005 at 8:30 AM E.T. It can be accessed by dialing 1-800-322-0079. International callers can dial 1-973-935-2100. You may also listen over the Internet at www.vcall.com. To listen to the live call on the Internet, please go to the web site at least fifteen minutes early to register, download and install any necessary audio software. For those who cannot listen to the live broadcast, a replay will be available approximately two hours after the end of the live call, through midnight (ET) on Tuesday, March 29, 2005. For replay, dial 1-877-519-4471 (1-973-341-3080 internationally). You will be prompted for PIN number 5802401.