“Fiscal 2004 was a year highlighted by the progress we achieved in our therapeutic programs, specifically in the Phase II double blind study of Alequel™, Enzo’s medicine for Crohn’s disease, and in the initiation of the Phase I/II study of our StealthVector® HGTV43™ gene construct, Enzo’s therapeutic for managing HIV-1,” said Barry Weiner, President. “The year was also marked by a transition in the marketing strategy of our Life Sciences division, and a major investment in the infrastructure of our clinical laboratory. With the additional investments in personnel, information technology and equipment, we continued to strengthen our core businesses and lay the groundwork for future growth. Our operating results reflect these investments and strategic initiatives that we believe will provide long term growth. Meanwhile, our financial resources remain strong, with in excess of $71 million in cash and cash equivalents, and marketable securities at fiscal year end, thus assuring our Company of a solid capital position to continue to strengthen the competitive position of Enzo Clinical Labs, capitalize on our leading developments in the life sciences and genomics area, vigorously pursue our promising therapeutics, and achieve what we anticipate will be the successful prosecution of infringements of our valuable intellectual asset base.”
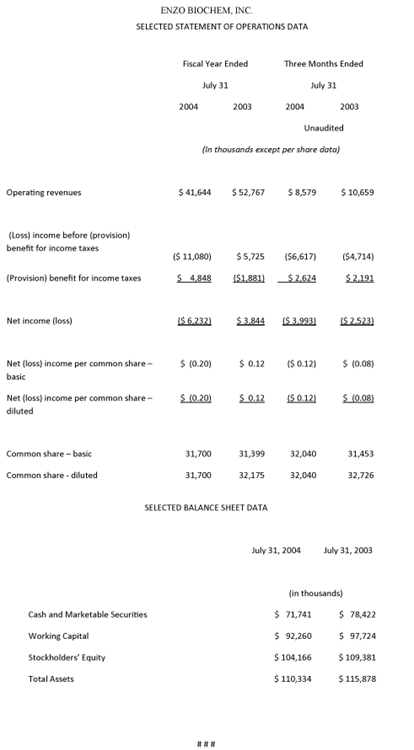
In the fourth quarter ended July 31, 2004, total revenues amounted to $8.6 million, compared with $10.7 million in the corresponding year-earlier period. Gross profits totaled $4.2 million, compared with $6.6 million for the previous fiscal year, and gross margins equaled 48.6%, compared with 61.5% in the fourth quarter of fiscal 2003. Reflecting a lower tax rate (39.7%, compared with 46.5% a year ago), the net loss for the fiscal 2004 fourth quarter amounted to $4.0 million, or ($0.12) per fully diluted share, compared with a net loss of $2.5 million, or ($0.08) per fully diluted share in last year’s fourth quarter.
For all of fiscal 2004, total revenues amounted to $41.6 million, compared with $52.8 million in the previous year, and gross profit totaled $28.5 million, compared with $39.8 million, with the gross profit margin at 68.5%, compared to 75.4% in fiscal 2003. The year’s net loss amounted to $6.2 million, or ($0.20) per fully diluted share, compared with net income of $3.8 million, or $0.12 per share, in fiscal 2003. Working capital at year-end totaled $92.3 million, and cash and cash equivalents and marketable securities amounted to $71.7 million, a decrease of $6.7 million. Last week, the Company’s Board of Directors declared a 5% stock dividend payable November 15, 2004 to shareholders of record on October 25, 2004.
Enzo Life Sciences posted fiscal 2004 revenues of $13.0 million, compared with $23.3 million in the prior year which included $11.5 million in sales to Affymetrix, Inc., whose contract was terminated by Enzo, and whose actions are the subject of a lawsuit against Affymetrix brought by the Company. “While our total sales declined for the year, it should be noted that, subtracting sales to Affymetrix from the fiscal 2003 total, revenues actually rose approximately 10% during fiscal 2004, reflecting the early stage in our program to shift sales from distributors to direct sales,” commented Mr. Weiner.
In the fourth quarter, Life Sciences revenue amounted to $2.0 million, compared to $2.6 million, in the corresponding year-ago period. The shortfall from the prior year’s quarter was due to the actions of one of its distributors, Roche Diagnostic Systems, in which revenues for products sold under an existing agreement were not recognized. In addition, the Company had a write-off in the amount of $1.8 million for a receivable from Roche due to Roche’s refusal to satisfy outstanding invoices from sales previously reported under that same agreement. The Company is currently in litigation with Roche.
This past year, Enzo Life Sciences completed development of line extensions for the BioArrayTM and GeneBeamTM products, which are now reaching the market, in addition to signing a supply agreement with GlaxoSmithKline related to Enzo’s proprietary RNA/DNA labeling, detection and amplification technology and products. The agreement includes a non-exclusive license under certain Enzo patents allowing Glaxo to generate genomic information for research and development activities. A patent covering methods of nucleic acid amplification was granted to Enzo by the U.S. Patent and Trademark Office. This patent is the first of several applications dealing with genetic testing, utilizing mutation analysis and SNP analysis, covering both linear and non-linear amplification.
At Enzo Clinical Labs, revenues for the year declined by 2.9% to $28.7 million, primarily due to recent downward trends in the reimbursement rates of third party payors and HMOs. In addition, the provision for uncollectible accounts receivables at the Labs increased by $1.5 million, which reflected a change in the mix of third-party payors. For the quarter, Enzo Clinical Labs had revenue of $6.8 million, versus $8.0 million in the fiscal 2003 period. The Labs posted a 5.2% gain in patient specimens for the year, and the expanded marketing effort, including added sales staff, has resulted in numerous additional physician clients in the Company’s expanded geographic reach. Additionally, the EnzoDirectTM System, Enzo Clinical Labs’ proprietary system for physician connectivity, continues to gain acceptance among doctors. “We believe that the investments made in the information technology and marketing at Enzo Clinical Labs during fiscal 2004 will produce improved results as we move forward through fiscal 2005,” said Mr. Weiner.
Legal expenses related to patent prosecutions and various lawsuits that Enzo has engaged in to protect its intellectual property estate totaled $6.3 million, compared with $5.7 million in the prior fiscal year. “This is a necessary expense to not only defend what is a valuable asset of the Company, but also to realize what we believe is monies that properly belong to Enzo,” said Mr. Weiner. Research and development expenses for the year remained essentially unchanged, amounting to $8.1 million, compared with $8.3 million in fiscal 2003.
At Enzo Therapeutics, Enzo recently reported that a Phase II randomized double-blind study of Alequel™, the Company’s investigational therapeutic modality for management of Crohn’s disease, successfully met its clinical endpoints. The Company also reported that an expanded study directed at a more diverse subject population and including dose escalation is in the regulatory approval process. This past year also saw initiation of the Phase I/II trial of Enzo’s proprietary StealthVector® HGTV43™ gene medicine to treat persons with HIV.
Other therapeutic activities included a Phase I clinical trial to study the safety of, a new immunomodulatory agent EGS21, a beta-D-glucosylceramide compound that could impact immune response by modulating its function. A paper on glucosylceramide was presented last May, suggesting that this drug could be an important candidate in the treatment of immune mediated diseases, such as Crohn’s, hepatitis B, hepatitis C and HIV. Enzo Therapeutics this past year also entered into two agreements with the University of Connecticut Health Center to license and cooperatively develop novel therapeutics for the stimulation and enhancement of bone formation, focusing on possible products that could provide therapy for bone disorders, including bone loss, fractures, abnormalities and various bone diseases. Additionally, Enzo entered into a licensing agreement with Thomas Jefferson University for certain patents relating to the development of Enzo’s Hepatitis B therapeutic program.
About Enzo
Enzo Biochem is engaged in the research, development and manufacture of innovative health care products based on molecular biology and genetic engineering techniques, and in providing diagnostic services to the medical community. The Company’s proprietary labeling and detection products for gene sequencing and genetic analysis, with over 200 patents worldwide, are sold to the life sciences market throughout the world. The Company’s therapeutic division is in various stages of clinical evaluation of its proprietary gene medicine for HIV-1 infection and its proprietary immune regulation medicines for hepatitis B and hepatitis C infection and for Crohn’s disease. The Company also holds a patent covering a method and materials for correcting point mutations or small insertions or deletions of genetic material that would allow for editing and correcting certain abnormalities in genes. For more information visit our website www.enzo.com.
Except for historical information, the matters discussed in this news release may be considered ”forward‑looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements include declarations regarding the intent, belief or current expectations of the Company and its management. Investors are cautioned that any such forward‑looking statements are not guarantees of future performance and involve a number of risks and uncertainties that could materially affect actual results. The Company disclaims any obligations to update any forward-looking statement as a result of developments occurring after the date of this press release.
An informational call conducted by Enzo Biochem, Inc. management will take place on Thursday, October 14, 2004 at 4:30 PM E.T. It can be accessed by dialing 1-877-780-2271. International callers can dial 1-973-582-2737. You may also listen over the Internet at www.vcall.com. To listen to the live call on the Internet, please go to the web site at least fifteen minutes early to register, download and install any necessary audio software. For those who cannot listen to the live broadcast, a replay will be available approximately two hours after the end of the live call, through midnight October 31, 2004. For replay, dial 1-877-519-4471 (1-973-341-3080 internationally). You will be prompted for PIN number 5274309.