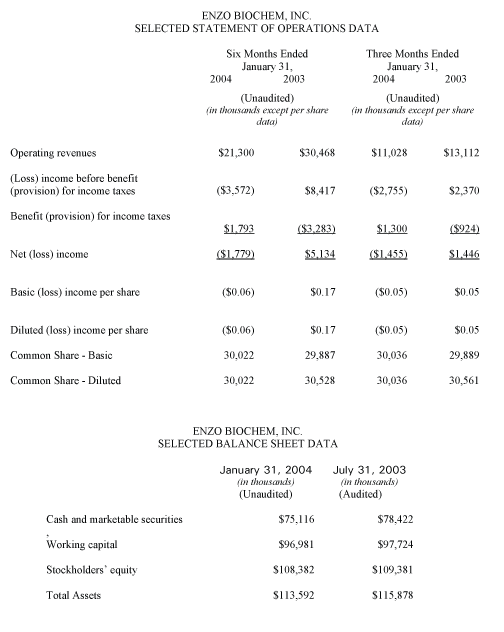
Reflecting principally the impact of decreased research products shipped by Enzo Life Sciences, operating revenue for the second fiscal quarter of 2004 amounted to $11.0 million, compared with $13.1 million in the corresponding year-ago period, and gross profit amounted to $8.1 million, compared with $10.4 million in the corresponding year-ago period. The net loss for the second fiscal quarter amounted to $1.5 million, or ($0.05) per diluted shared, compared with a year-ago net profit of $1.4 million, or $0.05 per share.
For the first fiscal half of 2004, operating revenue declined 30.1%, to $21.3 million. Gross profit for the six-month period ended January 31,2004 totaled $15.7 million, compared with $24.4 million in the corresponding year-earlier period. The fiscal 2004 first half net loss amounted to $1.8 million, or ($0.06) per diluted share, compared with a net profit in the corresponding year-ago period of $5.1 million, or $0.17 per share.
The balance sheet remained strong. At January 31,2004, working capital amounted to $97 million. Cash and cash equivalents, plus marketable securities, exceeded $75 million.
“Our Company continues to make progress,” said Barry W. Weiner, President. “Direct sales of research products, including our line of labeling and detection products for the genomics market, are increasing and the sequential performance is improving. Investments at Enzo Clinical Labs to further expand higher margin esoteric diagnostic tests are having a favorable impact. Enzo Therapeutics is currently conducting two clinical trials, and is preparing for multiple clinical trials for hepatitis B, hepatitis C, HIV, and Crohn’s disease, based on several of the Company’s therapeutic platforms. Preclinical research continues on a large number of other promising therapeutic modalities. We are concentrating on keeping expenses in line despite our broad scale activities in marketing, R&D and clinical investigation, as well as on the legal front, while remaining highly liquid and financially sound. We expect improved results in the periods ahead.”
In the second fiscal quarter of 2004, research and development expenses increased to $2.3 million, from $1.6 million in the corresponding year-ago period, due chiefly to the
investigational therapeutic trials underway. Selling, general and administrative expenses
increased $0.7 million, to $3.9 million, as the Company’s direct selling program aimed at the genomics market is being implemented. In the quarter ended January 31, 2004, legal expenses, which the Company views as an investment to protect its patent estate, amounted to $1.8 million, compared with $1.6 million a year ago, an increase of approximately 15%. Results benefited from a fiscal 2004 second quarter tax benefit, compared to a year-ago tax expense.
Revenues at Enzo Clinical Labs remained equal to a year ago, despite severe winter weather in its metropolitan New York marketing territory, which resulted in approximately 10 days during which specimen collections were unusually low. Higher priced esoteric tests increased during the period. The Clinical Lab represented 64% of total revenues in the fiscal 2004 second quarter, compared with 54.1% in the similar year-ago period. Gross profit was off slightly, to $4.5 million, compared with $4.8 million last year. The increase of approximately $1.1 million in the provision for uncollected accounts receivable reflects recent trends indicating decreased collection rates from certain third party payors, in addition to a change in the mix of payors. The Company continues to invest in new esoteric diagnostic equipment which is expected to further differentiate the Clinical Labs services in its highly competitive market, yield higher prices and benefit future results.
Enzo Life Sciences, in the second fiscal quarter of 2004, continued to be adversely affected by its decision to terminate a contract with Affymetrix, Inc., against which it has instituted legal action. The Company’s decision to field a direct sales force to market its proprietary labeling and detection products has begun to yield results. On a sequential basis, compared to the preceding first quarter of fiscal 2004, research product sales increased approximately 44%, and gross profit was $ 3.6 million, compared with $2.4 million. Subsequent to the second fiscal quarter of 2003, sales by the Company to Affymetrix ceased and, therefore future quarterly comparisons of our results should be more reflective of the Company’s continuing efforts.
Last week, Enzo Therapeutics reported early interim results for Alequel™, the Company’s investigational therapeutic modality for management of Crohn’s disease. To date, of the 19 evaluable subjects who completed the 15-week treatment period, with or without concomitant anti-inflammatory medication, 71% of those who received Alequel™ achieved clinical remission, compared to 25% of those who received a placebo. In addition, of those same subjects, 71% of those receiving the Company’s investigational therapeutic showed a clinical response, compared with 42% who received a placebo. Quality of life, an evaluation of health perception and function, showed a mean improvement of 45% in those subjects who received Alequel™, compared to an improvement of 9% among the placebo group. An expanded study is planned to enlarge the statistical base and to determine the duration of the effects of the treatment.
The Phase I/II study of Enzo Therapeutics’ Stealth Vector® HGTV43™ gene construct for HIV infection has been initiated at New York Presbyterian Hospital-Weill Cornell Medical College. In addition, plans for a multi-site Phase II study of EHT899, the Company’s immune regulation medicine for hepatitis B infection, are moving forward as an in-house capability to manufacture EHT899 is nearing completion.
Last month, Enzo reported that the Company acquired the assets of OraGen Corporation, a private biotechnology company specializing in immune regulation technologies. “This acquisition will broaden Enzo’s capabilities in developing immunological therapies for infectious diseases, including hepatitis B”, said Barry Weiner.
About Enzo
Enzo Biochem is engaged in the research, development and manufacture of innovative health care products based on molecular biology and genetic engineering techniques, and in providing diagnostic services to the medical community. The Company's proprietary labeling and detection products for gene sequencing and genetic analysis, with approximately 200 patents worldwide, are sold to the life sciences market throughout the world. The Company's therapeutic division is in various stages of clinical evaluation of its proprietary gene medicine for HIV-1 infection and its proprietary immune regulation medicines for hepatitis B and hepatitis C infection and for Crohn’s disease. The Company also holds a patent covering a method and materials for correcting point mutations or small insertions or deletions of genetic material that would allow for editing and correcting certain abnormalities in genes. For more information visit our website www.enzo.com.
Except for historical information, the matters discussed in this news release may be considered ”forward‑looking" statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements include declarations regarding the intent, belief or current expectations of the Company and its management. Investors are cautioned that any such forward‑looking statements are not guarantees of future performance and involve a number of risks and uncertainties that could materially affect actual results. The Company disclaims any obligations to update any forward-looking statement as a result of developments occurring after the date of this press release.
A conference call conducted by Enzo Biochem, Inc. management will take place on Tuesday, March 16, 2004 at 8:30 A.M. (ET). It can be accessed by dialing 877-692-2086. International callers can dial 973-582-2749. You may also listen over the Internet at www.vcall.com. To listen to the live call on the Internet, please go to the web site at least fifteen minutes early to register, download and install any necessary audio software. For those who cannot listen to the live broadcast, a replay will be available through midnight March 28, 2004, shortly following the live call. For replay, dial 877-519-4471 (973-341-3080 internationally). You will be prompted for PIN number 4585101.