The current global health crisis shines light on the systemic issues inherent in our healthcare system. From chasms within the supply chain to lack of readiness, staff and capacity, the Coronavirus pandemic has brought numerous inadequacies to the forefront. The central importance of the clinical laboratory as well as supply within the healthcare ecosystem has been diminished in recent years by government and regulatory bodies. While many believed there was an overcapacity of testing capabilities, the opposite has proven to be the case. The clinical laboratory is the one who must provide the testing, processing and detection/analytics for the entire market.
Based on the amount of diagnostic capital equipment in the marketplace, more than 20 million molecular diagnostic tests per day should be feasible within the United States. Closed diagnostic systems offered by the leading diagnostic companies have exacerbated the issues. These closed platforms provide little or no flexibility with regards to their workflow, enforce restrictive and limited supplier relationships, and have no direct connection with the patient or the event. These underlying problems have manifested themselves in the current COVID-19 crisis.
The Enzo Approach
We are offering molecular diagnostic testing, and serological “antibody” diagnostic testing for the novel Coronavirus, and are working on repurposing a drug candidate in our pipeline for the treatment of COVID-19. Enzo utilizes its technological and research and development capabilities, manufacturing infrastructure strength and clinical diagnostic knowledge to develop products that address gaps in performance, cost, obtainability and safety.
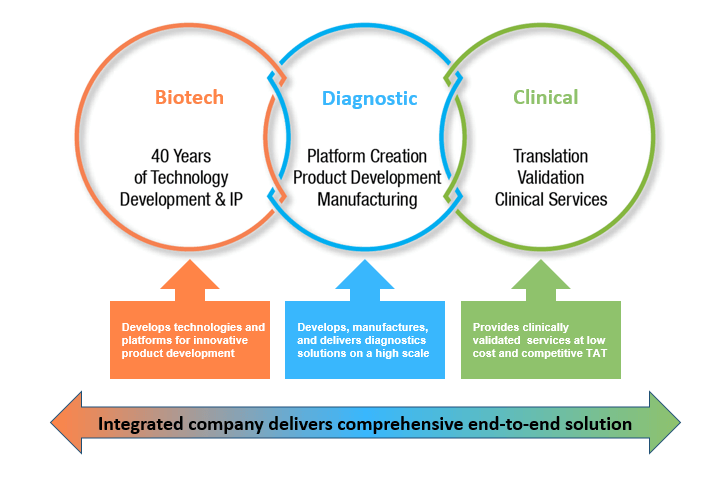
Enzo is one of few companies to incorporate a biotech entity, diagnostics division, and a CLIA certified clinical laboratory within the same company. Enzo has addressed challenges of the supply chain by manufacturing all of its critical reagents in-house. Enzo’s diagnostics equipment and kits are validated in its own lab prior to being released for sale in the marketplace for other labs and end users. Enzo is a fully integrated company with a comprehensive, end-to-end solutions for modern clinical diagnostics.
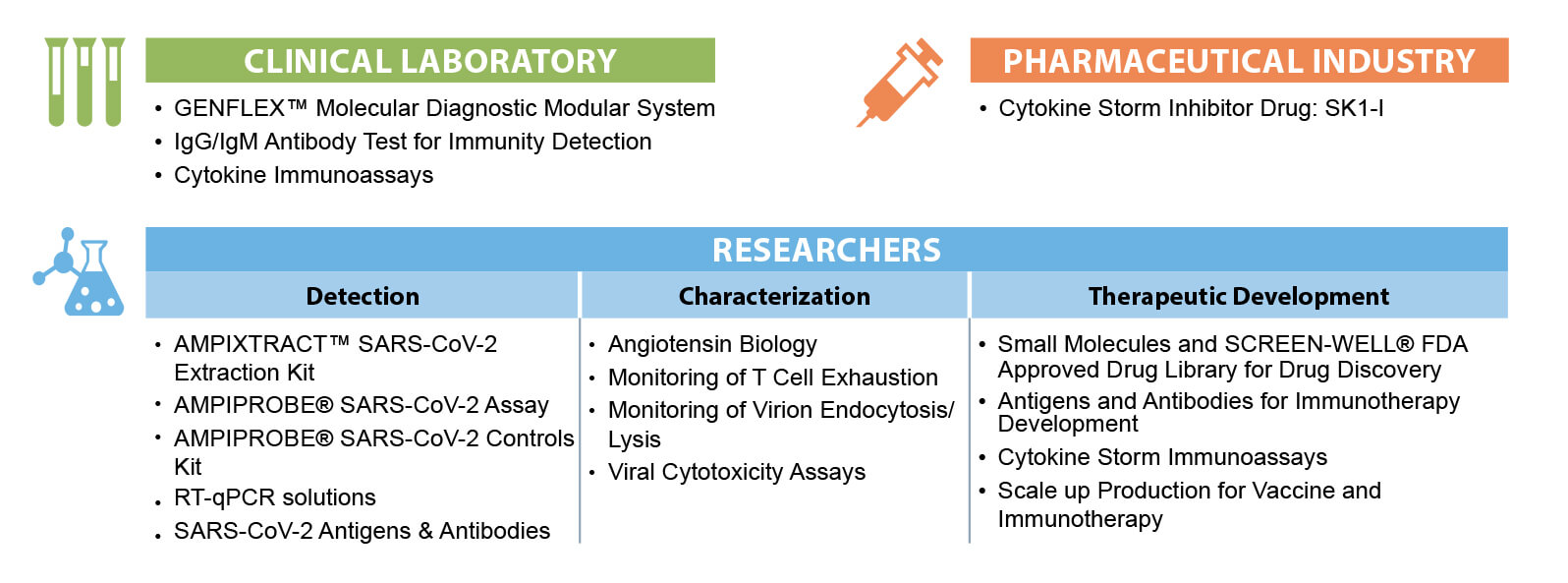
Molecular detection of the Coronavirus is the first line of defense in identifying infection. SARS-CoV-2 detection is performed using the GENFLEX™ Platform with its interlocking modules that include: AMPICOLLECT™ Collection Kit, AMPIXTRACT™ Extraction Kit, and AMPIPROBE® Detection Kit. Serological testing, while it should not be the sole basis for diagnosing COVID-19 can play an important role in identifying individuals who have overcome an infection in the past and developed an immune response. Detection of the body’s immune defense response – “antibody detection” – is performed using the Enzo IgG/IgM ELISA assay.
Infection with SARS-Cov-2 can cause the immune system to overreact and release inflammatory mediators to a detrimental extent in a process sometimes referred to as a “cytokine storm.” This response can be detected using Enzo’s ELISA cytokine storm assays. The Enzo patented SK1-I compound has been demonstrated to suppress the level of inflammatory cytokines in animal models of diseases such as lupus and in cytokine release assays performed with human cells ex vivo. Therefore, it is believed that SK1-I may hold promise for preventing and/or treating cytokine storm in COVID-19 patients. Enzo advances diagnostics by providing a complete picture of the SARS-CoV-2 virus through an integrated and comprehensive end-to-end solution backed by scientist enabling healthcare.