COVID-19 Multistep Approach
We are offering a comprehensive approach that tackles the disease from different angles. This includes molecular assays for the detection of nucleic acid from SARS-CoV-2, serological (antibody) assays for the detection of antibodies to SARS-CoV-2, tools for COVID-19 research and therapeutics, and clinical services to assist in the scalable testing of as many COVID-19 patients as possible. Molecular detection of the virus is the first line of defense to identify infection. Our AMPIPROBE® SARS-CoV-2 Test System allows for the molecular detection of SARS-CoV-2 viral RNA using the GENFLEX™ platform and its interlocking modules including: AMPIXTRACT SARS-CoV-2 Extraction Kit, AMPIPROBE® SARS-CoV-2 Assay Kit and AMPIPROBE SARS-CoV-2 Controls. We complemented our molecular test with the SARS-CoV-2 IgG ELISA Kit, a serological test for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum, and intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, including recent or prior infection. Infection with SARS-Cov-2 can cause the immune system to overreact and release inflammatory mediators to a detrimental extent in a process sometimes referred to as a “cytokine storm”. Enzo is in the early stage of development of a test for inflammation that may include IL-1 beta, IL-6, IL-8, TNF-alpha, and INF-gamma. This test is not available for use and is not FDA-cleared.
AMPIPROBE® SARS-CoV-2 Test System

The AMPIPROBE® SARS-CoV-2 Test System, which is comprised of the AMPIXTRACT SARS-CoV-2 Extraction Kit, the AMPIPROBE® SARS-CoV-2 Assay Kit and the AMPIPROBE SARS-CoV-2 Controls Kit, is a multiplex assay system based on real-time reverse transcription polymerase chain reaction (rRT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in upper respiratory specimens (such as nasal, mid-turbinate, nasopharyngeal, oropharyngeal swab specimens and nasopharyngeal wash/aspirate or nasal aspirate specimens) collected from individuals suspected of COVID-19 by their healthcare provider. The AMPIPROBE SARS-CoV-2 Test System has been authorized by FDA under an Emergency Use Authorization (EUA). It is adaptable to different extraction methodologies and throughput including our proprietary GENFLEX™ Platform, QIAsymphony® SP, and manual workflow.

Performance:
- Sensitivity*: 96.2%
- Specificity*: 98.0%
- Time to results: 192 samples in one shift
*Percent agreements between the AMPIPROBE® Test System and an FDA-approved EUA test performed on nasopharyngeal swabs
Our AMPIPROBE® SARS-CoV-2 Test System is designed to also be used in modular settings according to customer needs and consistent with the FDA EUA-approved directions for use. Each of the workflow components can be combined with any equipment specifically listed in the directions for use.
A. AMPIXTRACT™ SARS-CoV-2 Extraction Kit

The AMPIXTRACT™ SARS-CoV-2 Extraction Kit is part of our AMPIPROBE® SARS-CoV-2 Test System. It is designed for the isolation and purification of SARS-CoV-2 RNA virus from upper respiratory specimen (such as nasal, mid-turbinate, nasopharyngeal, oropharyngeal swab specimens and nasopharyngeal wash/aspirate or nasal aspirate specimens). Its magnetic bead separation technology enables high-quality purification of nucleic acids that are free of proteins, nucleases, and other impurities. The purified nucleic acids are ready for direct use in downstream applications, such as amplification and qualitative detection of SARS-CoV-2 virus.
RNA isolation can be performed with the AMPIXTRACT™ SARS-CoV-2 Extraction Kit either manually or via an automated process using Enzo’s GENFLEX™ platform.
B. AMPIPROBE® SARS-CoV-2 Assay Kit

The AMPIPROBE® SARS-CoV-2 Assay Kit is part of our AMPIPROBE® SARS-CoV-2 Test System and it is a multiplex assay designed for the detection of SARS-CoV-2 virus. It contains two primer/ probe sets specific to different SARS-CoV-2 genomic regions and primers/probes for internal control and negative control, human RNase P. It is adaptable to different extraction methodologies and throughput including our proprietary GENFLEX™ Platform, QIAsymphony® SP, and manual workflow.
C. AMPIPROBE® SARS-CoV-2 Controls

The AMPIPROBE® SARS-CoV-2 Controls is part of our AMPIPROBE® SARS-CoV-2 Test System and is intended to be used in combination with AMPIPROBE® SARS-CoV-2 Assay Kit and AMPIXTRACT™ SARS-CoV-2 Extraction Kit during the PCR process. The AMPIPROBE® SARS-CoV-2 Controls contains a positive RNA control that is specific to the SARS-CoV-2 genomic regions targeted by the assay as well as human RNase P, a negative RNA control that contains target to human RNase P, and a no-template control (NTC).
Emergency Authorization Use Only
In Vitro Diagnostic (IVD) Use Only
Prescription/Rx Use Only
The AMPIPROBE® SARS-CoV-2 Test System has not been FDA cleared or approved; the test has been authorized by FDA under an Emergency Use Authorization (EUA) for use by laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 U.S.C. §263a, to perform high complexity tests.
The AMPIPROBE® SARS-CoV-2 Test System has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
The AMPIPROBE® SARS-CoV-2 Test System is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
GENFLEX™ Platform
Our GENFLEX™ Platform is a high-throughput, automated, and scalable instrument for easy and accurate processing of common molecular diagnostic tests within a clinical production setting. Enzo has developed the specific reagents necessary for coronavirus detection that are compatible with this high-throughput platform. The AMPIPROBE® SARS-CoV-2 Test System combines the GENFLEX™ Platform with sample collection, extraction, and detection providing a flexible, yet accurate, approach for COVID-19 testing.
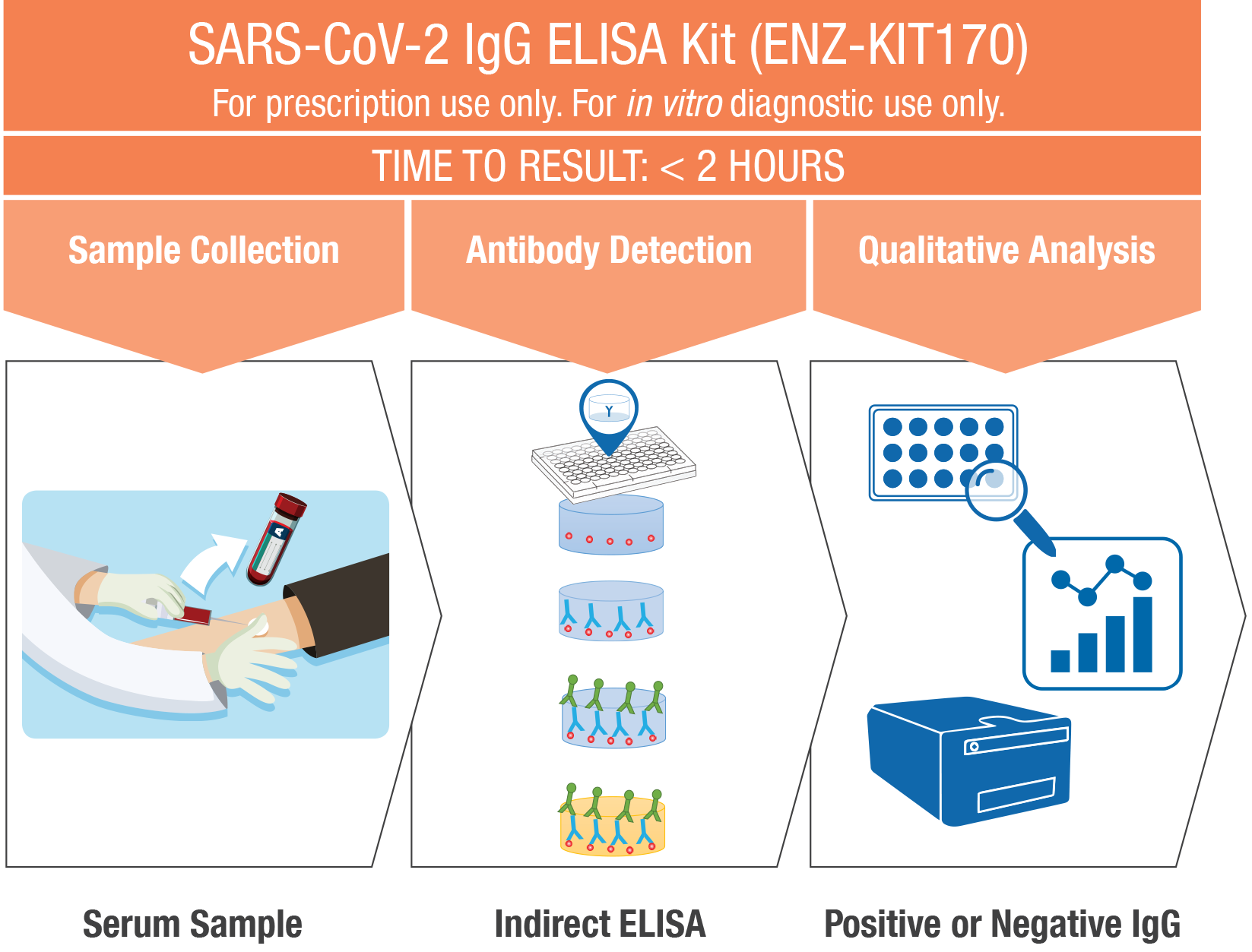
SARS-CoV-2 IgG ELISA Kit
While molecular tests serve as the frontline diagnostic tool to monitor SARS-CoV-2 active infections, serological tests are used to detect antibodies that are being produced by the patient’s immune system in response to the virus infection. Detection of the body’s immune response –antibody detection – is performed using the SARS-CoV-2 IgG ELISA Kit.
Our SARS-CoV-2 IgG ELISA Kit is an Enzyme-Linked Immunosorbent Assay (ELISA) designed for the qualitative detection of IgG antibodies specific to SARS-CoV-2 in human serum samples. This allows for scalable testing to deliver more accurate and more sensitive results than those obtained using the commonly available serology quick tests.
Designed for accurate and sensitive detection, the indirect ELISA has a two-step binding process involving a specific SARS-CoV-2 antigen and a HRP conjugated anti-human IgG secondary antibody
- Formatted in a 96-well microplate
- Adaptable to automated open platforms or manual workflows
- Easily scalable
Performance:
- Sensitivity*: 100%
- Specificity*: 96.5%
- Time to results: < 2 hrs
Contact Us for More Information
This test has been validated but FDA’s independent review of this validation is pending. This test is provided in compliance with FDA policy “Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised).” Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-2 infection. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
Cytokine Storm Monitoring
To manage a COVID-19 patient that tests positive, the following inflammation patterns will continue to be important. It is often not the direct effect of viral infection on cells that creates problems for the patient but the body’s immune response to this virus that is thought to contribute to the cause of death. Reported clinical experience suggests that uncontrolled release of cytokines causes a “cytokine release syndrome” or “cytokine storm” in some COVID-19 patients, which is associated with respiratory decline and failure. Accordingly, early monitoring of inflammatory cytokine levels has been suggested as an important diagnostic tool for determination of administering early therapy in managing these patients.
Enzo is in the early stage of development of a test for inflammation that may include IL-1 beta, IL-6, IL-8, TNF-alpha, and INF-gamma. This test is not available for use and is not FDA-cleared.